Abstract

Three novel C19 homolignans, designated taiwankadsurins A (1), B (2), and C (3), were isolated from the aerial parts of Taiwanese medicinal plant Kadsura philippinensis. The structures of 1−3, which have a 3,4-{1‘-[(Z)-2‘ ‘-methoxy-2‘ ‘-oxo-ethylidene]}-pentano(2,3-dihydro-benzo[b]furano)-3-(2‘ ‘‘-methoxycarbonyl-2‘ ‘‘-hydroxy-2‘ ‘‘,3‘-epoxide) skeleton, were determined by spectroscopic analyses, especially 2D NMR techniques (HMBC and NOESY). Compound 2 exhibited mild cytotoxicity against human KB and Hela tumor cells.(新骨架化合物)
Ya-Ching Shen*†, Yuan-Bin Cheng‡, Jun’ichi Kobayashi§, Takaaki Kubota§, Yohei Takahashi§, Yuzuru Mikami⊥, Junji Ito⊥, and Yun-Sheng Lin‡
School of Pharmacy, College of Medicine, National Taiwan University, Taipei, Taiwan 100, Republic of China, Institute of Marine Resources, National Sun Yat-sen University, 70 Lien-Hai Road, Kaohsiung, Taiwan, Republic of China, Graduate School of Pharmaceutical Sciences, Hokkaido University, Sapporo, 060-0812, Japan, and Research Center for Pathogenic Fungi and Microbial Toxicoses, Chiba University, Chiba 260-0856, Japan
J. Nat. Prod., 2007, 70 (12), pp 1961–1965
Abstract
Extensive column chromatography of the ethanolic extract of the soft coral Cespitularia taeniatacollected in Taiwan has resulted in the isolation of eight new nitrogen-containing verticillene diterpenes, designated cespitulactams D−K (1−8), together with three known diterpenes, cespitulactams A (9) and B (10) and cespitularin F. In addition, one new derivative, 6-O-acetylcespitulactam F (11), was prepared from compound 3. The structures of these compounds were elucidated on the basis of spectroscopic analyses, especially HRMS and 2D NMR experiments. The cytotoxicity against human oral epidermoid carcinoma (KB) and murine L1210 leukemia cell lines and antimicrobial activities of 1−8 and 11 were tested and evaluated. A biogenetic pathway for these novel diterpene alkaloids was also proposed.
J. Nat. Prod., 2008, 71 (4), pp 576–58
Abstract

Phytochemical investigation of the leaves and twigs of Taxus sumatrana afforded six new taxane diterpene esters, tasumatrols U−Z (1−6). Compounds 2 and 5 contained a rare five-membered lactone ring at C-8, C-9, C-10, and C-19. The structures were established on the basis of detailed spectroscopic analyses, particularly HRESIMS and 2D NMR techniques. Compound 5 showed cytotoxicity against a human hepatoma (Hep2) cell line.
J. Nat. Prod., 2002, 65 (7), pp 1052–1055
Abstract

Bioassay-directed fractionation of the methanolic extract of the leaves and flowers of Viburnum odoratissimum resulted in the isolation of two new diterpenes, vibsanol A (1) and vibsanol B (2), together with two new triterpenoids, 6β-hydroxylup-20(29)-en-3-oxo-27,28-dioic acid (3) and 6α-hydroxylup-20(29)-en-3-oxo-27,28-dioic acid (4). In addition, the known terpenoids vibsanins B and E, and 6α-hydroxylup-20(29)-en-3-oxo-28-oic acid, were also isolated. The structures of the new compounds were established by chemical and spectroscopic means. Vibsanol A (1) and compound 3 exhibited significant cytotoxicity against human gastric (NUGC) tumor cells.
Abstract

Investigation of an EtOAc-soluble extract of the soft coral Sarcophyton stolidotum resulted in the isolation of seven new 14-membered carbocyclic cembranes, sarcostolides A−G (1−7), together with two known cembrane diterpenes, isosarcophytoxide and isosarcophine. The structural elucidation of these metabolites was determined on the basis of spectroscopic analyses, particularly 2D NMR techniques. Sarcostolide E (5) exhibited weak to moderate cytotoxic activity against human WiDr and Daoy tumor cell lines. A biogenetic pathway and relationship for compounds 1−7 was also proposed.
Abstract

Phytochemical investigation of Eupatorium kiirunense has resulted in the isolation of eight new sesquiterpene lactones, constituted by five germacranolides, eupakirunsins A−E (1−5), and three heliangolides, eupaheliangolide A (6), 15-acetoxyheliangin (7), and 3-epi-heliangin (8), in addition to the known heliangin (9) and 8,10-epoxy-9-acetoxythymol angelate (10). The structures of the new compounds were established through detailed analysis of their spectroscopic data. Compounds 6, 8, and 9 exhibited cytotoxicity against human oral epidermoid (KB), cervical epitheloid (Hela), and liver (hepa59T/VGH) carcinoma cells.
Abstract

Chemical investigation of the soft coral Cespitularia hypotentaculata resulted in the isolation of six new diterpenes, cespihypotins Q−V (1−6). The new metabolites comprised five verticillane-type diterpenes and one nor-verticillane derivative. Their structures were determined through detailed spectroscopic analyses, especially HRESIMS and 2D NMR techniques. The relative configuration was deduced by interpretation of NOESY spectra. Cespihypotin T (4) exhibited significant cytotoxic activity against human Daoy and WiDr tumor cell lines.
Abstract

Bioassay-directed fractionation of the CH2Cl2−MeOH extract of Clavularia viridis collected in Taiwan has afforded seven new prostanoids, designated as 4-deacetoxyl-12-O-deacetylclavulone I (1), 4-deacetoxyl-12-O-deacetylclavulone II (2), bromovulone II (4), iodovulone II (5), 4-deacetoxyl-12-O-deacetylclavulone III (6), bromovulone III (7), and iodovulone III (8), in addition to seven known prostanoids (clavulones I, II, III, 7-acetoxy-7,8-dihydroiodovulone, chlorovulones II, III, and 4-deacetoxylclavulone II (3, claviridenone E)). The structures of compounds 1−8 were determined on the basis of 1D and 2D NMR techniques including COSY, HSQC, and HMBC experiments. Pharmacological study revealed that bromovulone III (7) and chlorovulone II exhibited the most promising cytotoxicity against human prostate (PC-3) and colon (HT29) cancer cells.
Ya-XuLina, Ahmed EidFazarya, Shun-YingChenb, Ching-TeChienb, Yao-HaurKuoc, Shiow-YunnSheud, Ya-ChingShen*,a
- Food Chemistry,
- Volume 123, Issue 4,
- 15 December 2010,
- Pages 1105-1111
Abstract
Three new prenylated C6–C3 compounds, illicaborins A–C (1–3), were isolated and characterised from the fruits of Illicium arborescens Hayata. Their structures were determined by extensive spectroscopic analyses (UV, CD, IR, 1H NMR, 13C NMR, 1H–1H COSY, HMQC, HMBC, and NOESY) including molecular modelling. Compound 1 possesses a new carbon skeleton having a prenylated C6–C3 skeleton with an additional CH2OH group at the C-2 position. The cytotoxic activities of compounds 1–3 were tested and evaluated against Hep-2, Daoy, MCF-7 and WiDr tumour cell lines.
12. New xenicane diterpenoids from
Tetrahedron Letters,
Volume 46, Issue 28, 11 July 2005, Pages 4793-4796
Ya-Ching Shen*, Yu-Chi Lin, Atallah F. Ahmed, Yao-Haur Kuo
Abstract
Three new xenicane diterpenoids, designated xeniolactones A (1), B (2), and C (3), were isolated from Xenia florida collected in Taiwan. Compound 1 possesses a novel structure having a heterotricyclic skeleton in cyclononane system. The structures of 1–3 were elucidated on the basis of extensive spectroscopic analysis. The cytotoxicity of 1–3 was also evaluated against human cancer cell lines.
Graphical abstract
Three new xenicane diterpenoids, xeniolactones A (1), B (2), and C (3) were isolated from Xenia florida collected in Taiwan.

13.Nitrogen-Containing Diterpenoids, Sesquiterpenoids, and Nor-Diterpenoids from Cespitularia taeniata (叢羽珊瑚)
by Shih-Sheng Wang,Yuan-Bin Cheng,Yu-Chi Lin,Chia-Ching Liaw,Jiun-Yang Chang,Yao-Haur Kuo and Ya-Ching Shen*Mar. Drugs 2015, 13(9), 5796-5814
Abstract
Two new nitrogen-containing verticillene diterpenoids, cespilamides A and B (1 and 2), three new nitrogen-containing sesquiterpenoids, cespilamides C–E (3–5), and five new norverticillene and verticillene diterpenoids, cespitaenins A–E (6–10), were isolated from the Taiwanese soft coral Cespitularia taeniata. Compound 1 possesses an unusual oxazo ring system at C-10 while compound 2 displays an unprecedented C–C bond cleavage between C-10 and C-11 with an N-ethylphenyl group at C-10. Biogenetic pathways of 1and 2 are proposed. The absolute configuration of 1 was confirmed by Mosher’s method and molecular mechanics calculations (MM2). The cytotoxicities of compounds 1–10 were evaluated against a small panel of human cancer cell lines.
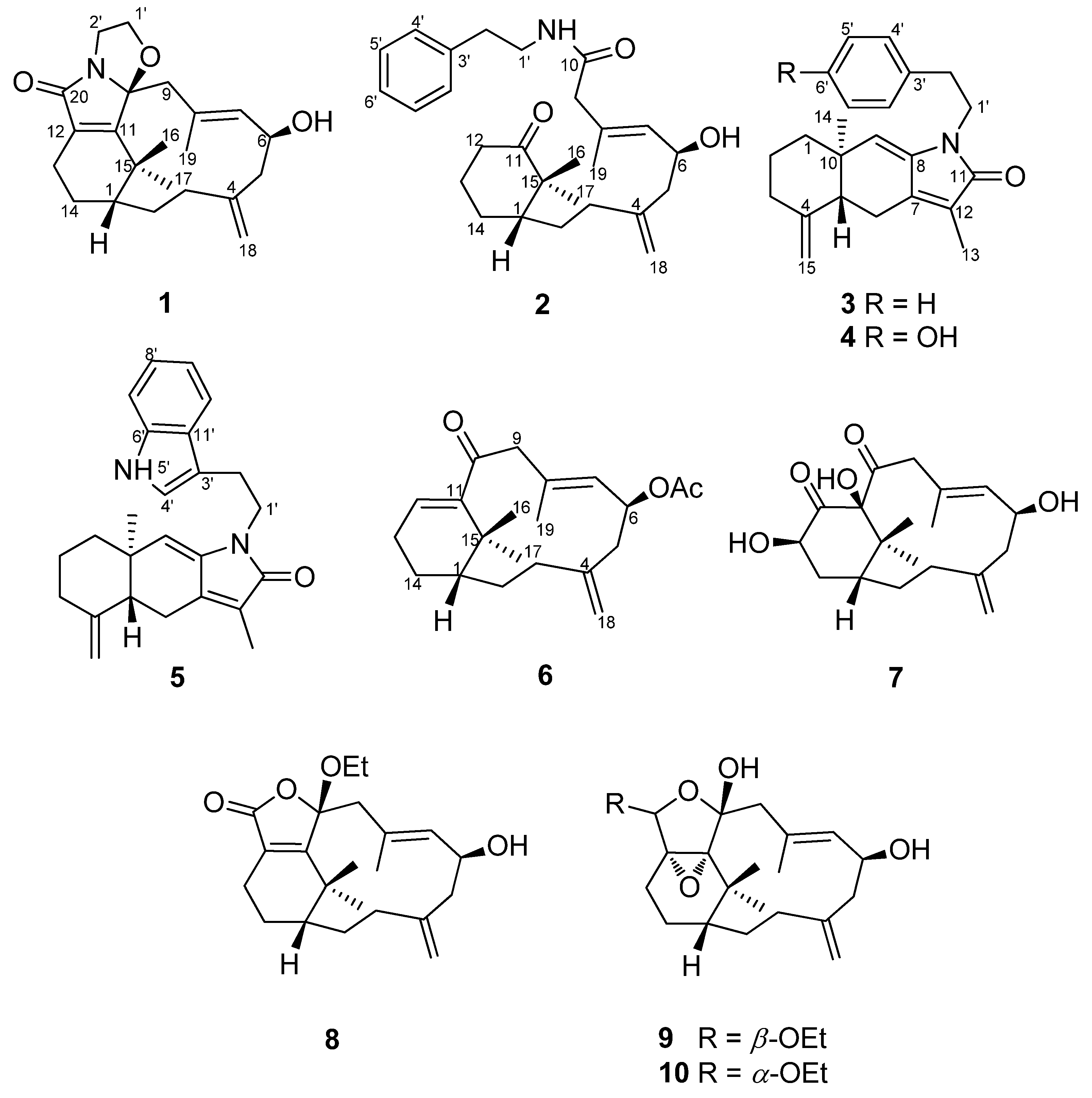

Abstract
15. abeo‐Taxane Diterpenoids from the Taiwanese Yew Taxus sumatrana (台灣紅豆杉)
Shih‐Sheng Wang, Mohamed H. Abd El‐Razek, Yi‐Cheng Chen, Ching‐Te Chien, Jih‐Hwa Guh, Yao‐Haur Kuo, Ya‐Ching Shen*
Chemistry & BiodiversityVolume 6, Issue 12
2153-2392
December 2009
Abstract
Chemical investigation of the acetonic extract of the leaves and twigs of Taxus sumatrana (Taxaceae) led to the isolation of four new taxane diterpene esters, taiwantaxins A–D (1–4, resp.). Their structures were determined primarily on the basis of 1D‐ and 2D‐NMR techniques as well as MS. Compound 1 is a rearranged taxane diterpenoid possessing an opened oxetane ring moiety containing C(4), C(5), and C(20). The metabolites 2 and 3 belong to a 5/6/6 taxene system having a rare five‐membered γ‐lactone ring comprising C(8), C(9), C(10), and C(19). Compound 4 is an example of a taxane diterpene containing a 6/8/6 ring system with a tetrahydrofuran ring comprising C(2), C(3), C(4), and C(20). The 11(15→1)abeo‐taxane diterpenoids, taiwantaxins A–C (1–3, resp.) are lacking an O‐bearing functionality at either C(13) or C(14). Compound 2 showed significant cytotoxic activity against human PC‐3 tumor cells.
16. Cembrane diterpenoids from the Taiwanese soft coral
Tetrahedron, Volume 65, Issue 45, 7 November 2009, Pages 9157-9164
Yun-Sheng Lin, Chung-Hsiung Chen, Chia-Ching Liaw, Yu-Chen Chen, Ya-Ching Shen*
Abstract
Chemical investigation of the soft coral Sinularia flexibilis (Quoy and Gaimard), collected from the southern coast of Taiwan, led to the isolation of 10 new cembrenoid diterpenoids, the flexilarins A–J (1–10), along with 17 known compounds (11–27). The structures of these compounds were elucidated by spectroscopic techniques (NMR, MS, UV, IR). The structure of compound 1 was confirmed by X-ray crystallographic analysis. Compound 4 exhibited potent cytotoxicity against Hep2 tumor cells.
Graphical abstract
Chemical investigation of the soft coral Sinularia flexibilis led to the isolation of 10 new flexilarins A–J (1–10). Compound 1 was confirmed by X-ray crystallographic analysis. Compound 4 showed potent cytotoxicity against Hep2 tumor cells.

17. Cespitulactams A, B, and C, three new nitrogen-containing diterpenes from
Tetrahedron Letters,
Volume 46, Issue 46, 14 November 2005, Pages 7893-7897
Ya-Ching Shen*, Yun-Sheng Lin, Yao-Haur Kuo, Yuan-Bin Cheng
Abstract
Three new nitrogen-containing diterpenoids, designated cespitulactams A (1), B (2), and C (3), were isolated from Cespitularia taeniata May. Compounds 1–3 are novel structures having a phenylethyl amino side at C-10 and with an amide function at C-20. Their structures were determined on the basis of extensive spectroscopic analysis and chemical correlation. The cytotoxicity of 1 and its monoacetate (6) were also evaluated against human cancer cell lines.
Graphical abstract
Three new nitrogen-containing diterpenoids, designated as cespitulactams A (1), B (2), and C (3), were isolated from Cespitularia taeniata May.


